Balancing High Quality with Stable Quality

Our Quality Policy requires that we provide a stable supply of high-quality products. As society progresses, customers' quality demands are becoming more sophisticated and diverse. In order to respond to these changes, each of our plants has set its own management standards that exceed the quality standards according to the characteristics of the products. We are committed to continuous improvement and advanced quality control.
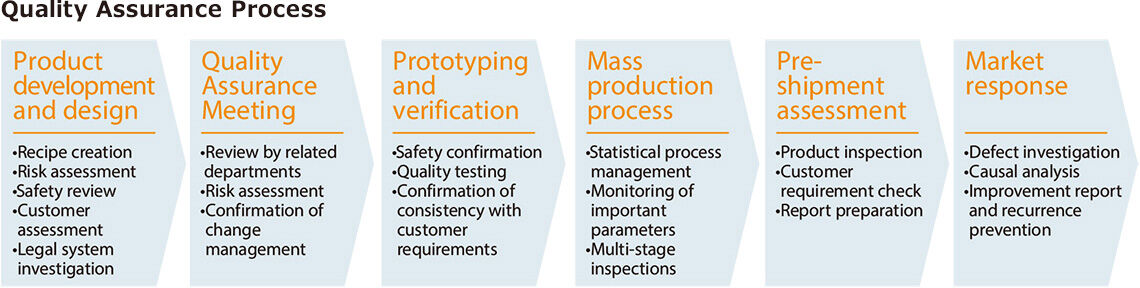
Quality Assurance Process
We prioritize safety and stability from the development stage, developing designs and recipes based on risk assessments. Prior to production, we hold Quality Assurance Meetings and conduct multifaceted reviews, including risk assessments and change management, with collaboration between relevant departments. During the prototyping and validation stages, we verify consistency with customer requirements through quality testing and safety confirmation. During mass production, we combine statistical process management, monitoring of key parameters, and multi-stage testing to flexibly address raw material variations and external factors. Furthermore, we have established a comprehensive analytical system, from raw material receipt to pre-shipment inspection, to check customer requirements. In the unlikely event of a defect occurring in the market, we promptly investigate and analyze the cause, implement corrective measures, and report them to prevent recurrence. Furthermore, we raise employee awareness through training, human resource development, and international standard certifications. By incorporating customer feedback and requests, we continually provide reliable quality.
Quality Assurance Meeting
At each of our plants, we hold Quality Assurance Meetings at the production design stage to review processes to ensure safe manufacturing with stable quality. The meetings are led by the quality assurance department and are attended by representatives from departments related to the products being produced. Production begins only after quality and safety have been confirmed, with processes reviewed based on checklists defined in the manual.
Initiatives for Products Safety and Stable Quality
Photosensitive Materials Business
The Chiba Plant primarily manufactures cutting-edge semiconductor materials, placing emphasis on ensuring high quality and safety. Quality Assurance Meetings are held for review from the development stage, and strict risk assessments are conducted for any process changes. All products are also subjected to detailed inspections using various analytical equipment, and advanced analysis and statistical quality control are carried out. In the event of an abnormality, information is promptly disclosed to customers, control standards are strengthened upon request, and we work together to improve quality levels.
Chemicals Business
We mainly manufacture purity solvents used in the production of electronic materials and ingredients used in flavorings and fragrances. With our basic policy "build quality into products," we strive to achieve both high quality and stable quality in all processes, from product development to manufacturing, inspection, and shipping. At the product development stage, we design optimal products according to their intended use. We also work to develop manufacturing processes that meet the ever-changing needs of our customers, who are striving for high quality.
In the manufacturing process, we have detailed procedures for each step, from checking the receipt of raw materials to shipping inspections. We carry out strict monitoring and inspections based on these procedures.
For electronic material solvents, we have established technology to manage and analyze metal ions at the parts per trillion (ppt) level, allowing us to provide high-quality products based on highly reliable analytical data. For fragrance ingredients, odor, evaluated by human judgment--is the most important factor in quality. Therefore, in addition to standard instrumental analysis, we also conduct sensory testing (evaluation using human olfactory sense) to ensure that we deliver fragrance ingredients of consistent quality that consistently reproduce the same fragrance characteristics. Furthermore, we share customer feedback throughout the plants and strive for continuous quality improvement using our quality assurance system in which all employees participate.
Logistics Business
Takahama Chemical Tank Terminal handles the logistics of liquid chemical products entrusted to us by our customers. The facility implements thorough management under a quality assurance system unique to a chemical manufacturer. When filling stored products, we use high-performance filters suited to the type of oil and filling capacity to prevent contamination by foreign matter, and in addition to analysis at the time of receipt and sampling at the time of shipment, we also carry out additional analysis according to customer needs. We are equipped with advanced analytical equipment to deal with risks specific to chemicals, such as quality deterioration and contamination. We also carry out thorough quality control during storage and loading and unloading operations, striving to improve customer satisfaction. We can flexibly handle a variety of cargo handling methods, from drums to tanker trucks and ships, and also handle a wide range of special transfer operations, such as refilling from drums to tanker trucks and receiving and transferring between tanker trucks and ISO containers. Our automated multi-story warehouse can accommodate up to 10,000 drums. We actively work to reduce the amount of exhaust gases that lead to air pollution, such as PRTR target substances and foul-smelling substances, thereby achieving safety, high quality, and environmental considerations all at once.
Main Initiatives to Prevent Defects from Occurring
Due to the characteristics of photosensitive materials, the mainstay product of our Photosensitive Materials Business, quality defects that cannot be detected by ordinary chemical analysis can occur. In some cases, it is difficult to identify the cause; thus, we identify all possible causes and implement preventive measures. For manufacturing processes that pose a particularly high risk of causing defects, we establish strict control parameters and appropriate manufacturing procedures, and manage them thoroughly. When making changes to processes, we generally notify customers 12 to 18 months in advance. After that, we conduct reviews at Quality Assurance Meetings, conduct thorough risk assessments, and obtain customer approval before making any changes.
Furthermore, we place great importance on change management training to align employees' understanding of process changes so that all employees can act with the same mindset, including recognizing the risks of process changes and determining what constitutes a change.
In our Chemicals Business, we prioritize identifying and addressing defects at an early stage. In addition to strict inspections when raw materials are received and parameter management in the distillation process, we always identify risks and consider countermeasures using Failure Mode and Effects Analysis (FMEA) when making process changes, and major changes are deliberated across departments. We also provide thorough change management training so that all employees can make decisions based on the same standards. Furthermore, we conduct product recall training once a year in collaboration with plants, quality assurance, sales, and the supply chain. We have a system in place to respond quickly in the event of an emergency, thereby preventing defects from occurring.
Chemical Substance Management
As a chemical manufacturer, we have established our own chemical substance management regulations to ensure the safe handling of chemicals and to minimize their impact on the human body and the environment. Each of our business locations complies with relevant domestic and international laws and regulations and strives to properly manage the chemical substances it manufactures and sells.
When introducing new raw materials or products, we conduct risk assessments based on toxicity and environmental impact, and confirm their safety before supplying them to the market. We also create Safety Data Sheets (SDS) for all products and display GHS labels on product containers and packaging to accurately communicate toxicity information.
We also provide regular education and training to ensure that our employees have a correct understanding of chemical substances and can manage them safely. For example, at the Aroma Chemicals Plant, we create an SDS summary sheet for each substance so that employees can understand the contents of the SDS and refer to it whenever necessary. This sheet summarizes the following points: 1) physical properties, 2) GHS classification, 3) relevant laws and regulations, 4) emergency response, 5) examples of workplace accidents, and 6) risk assessment. It is posted in the analysis room and available for constant reference. We will continue to promote initiatives that anticipate regulatory trends and societal demands, aiming to enhance chemical substance management and contribute to a sustainable society.
Export Control
Some of our products incorporate advanced technologies and are subject to security trade control regulations. The Company has established export control regulations approved by the Ministry of Economy, Trade and Industry and operates a company-wide export control system under the responsibility of the President to ensure proper export management.
- Message from CEO
-
TOYO GOSEI REPORT2025 DOWNLOAD
(Japanese version only) - Toyo Gosei Co., Ltd. in Our Lives
- Toyo Gosei's Business and Sustainability
- Safety Initiatives
- Human Capital Initiatives
- Initiatives for Respecting Human Rights
- Social and Environmental Initiatives
- Initiatives for Business Continuity
- Initiatives for Sound
Corporate Management - Research and Development
- Sustainability Data

